The dietary, lifestyle, and environmental changes we can make give us great hope in improving our quality of life in the face of autoimmunity. But what is the role of DNA? Are we predestined to suffer because of certain gene mutations? Experts say that our DNA is only 30% of the equation in autoimmunity, and that “our DNA is not our destiny” – meaning that just because we have certain genes does not mean that a disease will automatically turn on. Instead, it means that if the conditions are set for disease by diet, lifestyle, and environmental factors, then the genes for that disease can be more easily triggered.
So is DNA even worth looking at when we consider autoimmunity? Is there anything more that can be done to reduce the risks of activation?
The answers are Yes, and Yes.
DNA and Epigenetics
Most people have a core understanding of what genetics is, being the strands of DNA in the nucleus of each cell, half of which were donated from their mother and half from their father. (2) DNA is formed in a double helix, with one gene on each side, and these genes are formed into chromosomes, or paired X-shaped tubes of DNA folded in upon itself.
The individual gene segments themselves are referred to as SNPs and each SNP, or combination of SNPs, contains the code for the production of an enzyme, protein, neurotransmitter, or other substance needed for the cell and for the body as a whole. Each cell in your body contains the same base DNA, though that DNA is expressed differently for different types of tissues, and serves a different function.
Epigenetic shifts occur when a gene is turned “on” or “off,” altering its expression and overall function. Many genes in each of our cells are normally “off,” such as the genes that code for digestive enzymes in your big toe. Other genes are normally “on,” such as those same digestive enzymes in your gut. Many thing — and it could be theorized that all things that interact with the body — can act as epigenetic triggers, making certain genes switch between off and on. An easy way to understand this process is to think of genes as always having the same information stored, like the components in light switches. Epigenetics is the study of how those switches get flipped, and what happens when they do. (3)
We’re going to highlight some ways in which your epigenetic shifts can change your health for the worse, or for the better. You’ll begin to realize that you have much greater control over your health than you thought and, as you learn more, you can apply this knowledge to be proactive and increase your health and the health of those for whom you care.
Practical application of genetics
Genetics can only assess risks for certain diseases. The epigenetic factors in a person’s life make up the other part of that risk, which can either increase or decrease overall chance for the disease. A good example of this is the BRCA1 gene, which impacts breast cancer risk. Those who have a variant of the BRCA1 gene have a significantly increased risk of breast cancer. BRCA1 is a gene that suppresses tumor growth, so it makes sense that an impaired gene variant would increase the chances of developing cancer.
The increased risk, however, seems to be getting higher as the world becomes more industrialized. There are studies that show the correlation of increased estrogenic and xenoestrogenic compounds in foods and body care products, as well as of the increased electromagnetic waves we’re exposed to on a daily basis being linked with tumor development in people with the BRCA1 mutation. More tribal nations, on the other hand, while they may have the same mutations, show only a slightly increased rate of breast cancer development compared to the rest of the population. There are more factors than these that can impact the behavior of this gene and the health of those who have it, but these environmental influences appear to be one of the largest factors. So it stands to reason that reducing these environmental toxins and exposures will again reduce risk. (5, 6, 7)
The BRCA1 example is one of the more straightforward cases of epigenetics playing a role in health. Autoimmunity can be more complex, as it usually involves more than one gene with multiple effects involved, but the principle is the same. (4)
Autoimmunity describes when the body’s immune system attacks its own tissues. There are over 80 named autoimmune conditions, from Hashimoto’s thyroiditis, to rheumatoid arthritis, to multiple sclerosis. Each named condition attacks specific types of tissues in the body, and it’s possible to have multiple of these conditions at the same time. It’s interesting to note that the beginning stages of some other major disease conditions begin as autoimmune reactions as well, such as heart disease and cancer. Because these types of conditions can be so closely linked, it makes sense to moderate our immune reaction to decrease our risk for many diseases. Epigenetically speaking, it’s the mechanisms of turning these genes “on” that matter more than the specific genes themselves. (10)
Researcher and physician Dr. Tom O’Bryan, DC, CCN, DACBN, likens the genetic potential for disease to a chain. If you continually pull on the chain, choosing lower quality foods, being sedentary, or dealing with a great deal of stress, that chain is going to break at its weakest points. Those vulnerable spots in the chain are the genes that show potential for disease development. So, autoimmune conditions occur when the chain keeps getting tugged on, over and over, without allowing those weak points to tighten back up. The first thing he suggests is to stop pulling on the chain, so to speak, by changing the factors in our life that may be weakening it, such as controlling inflammation and calming the immune response of our body. After that, we can begin to look at specific therapies to begin healing the body — though it’s important to remember that once snapped, those links on the chain are unlikely to join back together. (14)
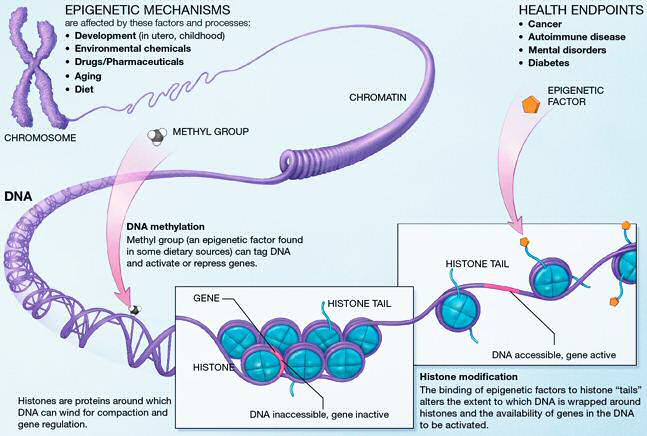
Image Credit By National Institutes of Health – http://commonfund.nih.gov/epigenomics/figure.aspx, Public Domain, https://commons.wikimedia.org/w/index.php?curid=9789221
One of the most intensely studied sets of epigenetic shifts occurs due to variants in the MTHFR genes. It’s so often referenced only by its acronym that some researchers are challenged to remember its full name, Methylene Tetrahydrofolate Reductase, which is a mouthful in any circumstance. These genes code for the enzyme of the same name. Let’s look at a brief overview of what these genes control.
MTHFR mutations
There are two primary genes involved in MTHFR enzyme production: C677T and A1298C. (1) The mutations that can occur on these genes can change a person’s entire methylation process, making it more difficult to absorb certain nutrients, and making some nutrients that would otherwise be beneficial actually harmful to ingest. More than that, since epigenetic shifts are dependent on methylating the genes involved in each process, those of us with these mutations are more prone to changes downstream that can trigger further disease. Below you can see how much of this enzyme is produced in those of us with various degrees of mutation.
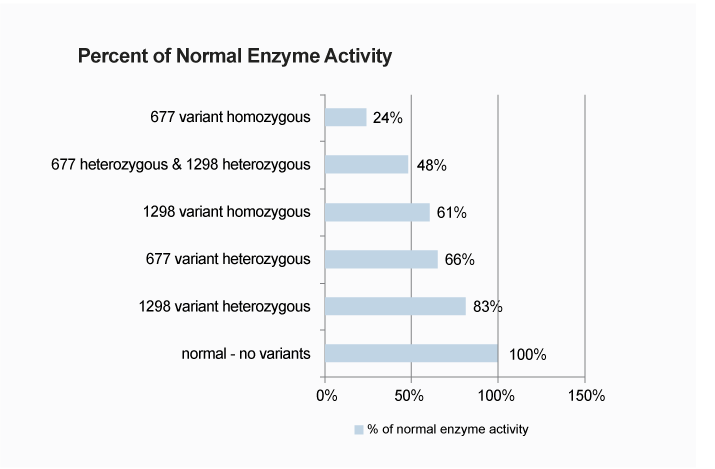
Image credit http://www.iversongenetics.com/mthfr.html
The MTHFR mutations have been linked to an impaired detoxification ability, as well as the development of over 60 other diseases. Because it’s so common, those of us who have this mutation must be smart about our dietary and lifestyle choices. These are the key to keeping the rest of the genome expressing effects that are positive for our overall health. Our goal is to support the impaired processes to make up for the mutation. (11, 12)
Methylation is also important for the conversion of homocysteine to methionine, which is then converted to your body’s master antioxidant and detoxifier, glutathione. When methylation is impaired due to genetic variations or epigenetic changes, these conversions become inefficient, and symptoms begin to surface. (13)
Those of us with any MTHFR mutations are recommended to supplement with pre-methylated forms of vitamins, such as methylfolate (as opposed to the folic acid found in many fortified foods, which can cause problems for individuals with MTHF mutation) and methylcobalamin, which is a form of B12 that is more easily absorbed.
Why are MTHFR mutations so strongly linked to those with autoimmune conditions? The reason goes back to impaired detoxification. In this case, those of us with these mutations have a much harder time detoxing heavy metals we come into contact with. Heavy metals such as mercury and lead are surprisingly pervasive in our air and food supply, and the buildup of these heavy metals in the body interferes with the function of our immune system. It seems that thyroid conditions are the most common result, such as Hashimoto’s Thyroiditis, but others are also seen regularly, such as rheumatoid arthritis and scleroderma.
As you can see, it’s important to account for these mutations, if you have them, in your daily choices. Some steps you can take to keep yourself healthy include avoiding fish that are high in heavy metals, such as tuna. Smaller fish tend to have less mercury, since they are caught earlier in their life cycle and have had less time to accumulate mercury in their bodies. If you live or work in rural areas, your air quality is typically cleaner, but if you are in the suburbs, in the city, or near factories, you could consider high quality air and water filters that will remove toxins that could cause illness.
Maybe you’ve known for a long time that something was just “off” and you couldn’t pinpoint it to any specific food, supplement, or activity. Looking into your specific methylation genes can be a great starting point to formulate a personalized plan with a functional medicine practitioner, since each of us has slightly different needs.
Decoding mystery
One of my clients likens it to having known her whole life she couldn’t eat like other people. She always did eat like other people because she didn’t know what she needed to do differently. In her case, she ate a standard American diet, with plenty of chips, cookies, and ice cream. Over the years, she figured out some things that worked and some things that didn’t; which foods made her feel great, and which ones made her groggy, stiff, and bloated. After we worked together on what was best for her based on her genome and the epigenetic changes she experienced early in life, she adopted a way of eating perfectly tailored to her body.
We learned that she has heterozygous mutations in both of the major MTHFR genes, which means that each gene has one normally functioning side, and one that does not work as well. Because of this, she no longer eats grains or dairy, and focuses on fresh meats and vegetables, with a few specific supplements like methylfolate, curcumin, and magnesium.
Now, she feels amazing every day, and staying healthy and in shape is almost effortless because she has the energy to move and she’s able to process information far faster than she used to. Her inflammation is dramatically reduced, and she says she feels like an entirely new person.
She’s right about that. Since our cells are all replaced periodically, she has what amounts to an entirely new body from when she began her journey into health. With each positive step that she made, the next generation of cells was healthier, stronger, and in some cases younger, as markers of aging tend to decrease with mindful actions towards better health. And with what we’re learning about autoimmunity today, these positive changes in her diet and lifestyle have no doubt prevented the triggering of other disease that could have easily turned on had her eating habits remained as they had been.
One of the things this particular client had as an advantage in was her mindset. She had already known all her life that her way of eating had to change, so once she had the right information about what to change, that shift was an easier adjustment. For a lot of us who have to change our way of eating to heal and stay healthy, adjusting habits and preferences can be a challenge, especially when we have to eliminate staples of their diet or longtime favorites. That’s where a health coach or partner for the journey can be a big help. Pop over to our resource directory and social network to get some help, or learn more about how to make positive change in our Lifestyle module.
What if an epigenetic switch gets turned on?
Once a disease-promoting epigenetic switch has been turned “on,” you can’t exactly turn it off again. Most people I work with come to me because their disease promoting genes have already been triggered, and they are having symptoms that they would like to resolve. If you understand your genome before your genes are triggered into creating illness, you have an advantage in that you can make positive changes sooner to avoid the diseases that you may otherwise be prone to. But if the disease has already been put in motion, making positive changes in your diet and lifestyle can have significant positive effect, and arrest and even sometimes reverse the course of disease. You’ll learn more specific examples of what you can discuss with your functional medicine practitioner in our Diet and Lifestyle modules.
Dietary impact on genetic mutations
What are some of the things that can epigenetically shift genes? The list of these, if we want to get specific, can be exhaustively long, because our genes can react to any number of factors. The primary way we can both accidentally and deliberately switch our genes “on” or “off” is by our food choices. A diet that is low in nutrients but high in carcinogenic compounds, such as many fried and processed foods, can switch off the genes that lead to a long and healthy life, and turn on genes that promote the growth of tumors, impair circulation, harm detoxification, increase inflammation, and cause autoimmunity, among other disease states. A diet full of foods that contain the vitamins and minerals that support health in general, along with antioxidants, phytonutrients, and other compounds that are beneficial tend to turn on genes that improve health and longevity, and that can fight off diseases more effectively, while turning off the genes that promote those disease states. In this way, you can see that diet plays a two-fold role: the foods themselves promote either health or disease, and their interaction with your genes amplifies that effect.
A poor diet that causes inflammation in the digestive system can increase intestinal permeability, which means that small particles of undigested foods can leak out into the bloodstream. These particles are seen as pathogens by the immune system, which then produces antibodies against them in response. It is now understood that intestinal permeability or “leaky gut” is one of the key prerequisites for autoimmune disease to turn on.
Environmental impact on genetic mutations
Foods aren’t the only thing that impact your epigenetic triggers, though. We are electrical beings, and all of our cells rely on specific electrical triggers to fire, from neurons in the brain to the muscles of the heart. All of our cells are electrically charged, and this includes the particles that make up your DNA. In today’s high-tech world, we are constantly bombarded by electromagnetic waves, from wi-fi antennas, to cell phone signals, to even radio waves. Our highly charged atmosphere can play a role in epigenetic changes has been observed especially in children, in the delicate tissues of the brain. Increased electromagnetic exposure has been linked to deterioration in the ability to form short and long term memory and impairs cognitive ability. This effect was observed on a genetic level, where the genes produced imbalanced levels of the neurotransmitters that foster healthy development. One study even showed that low level EMF exposure, which we’re exposed to every day, can alter methylation over the entire genome, which means it can have any number of downstream effects. (8, 9)
Previous bacterial or viral infections can cause changes to the way genes express themselves. Infections are such a pervasive trigger because of the way the immune system reacts to them. Many infectious microbes contain proteins that are remarkably similar to our own, and the antibodies that our immune system produces begin to see our own tissues as pathogens. Toxins like mold and fungus are also able to epigenetically shift our genes. You’ll learn more about these in our Gut and Environmental modules.
The impact of stress on genetic mutations
Stress, because it alters immune function, can be a trigger for autoimmune disease if the stressed state is intense or lasts a long time. Stress isn’t just a mental state. Instead, it’s a broad term for our biological reaction when we feel like we’re in danger. This can include a dramatic hormone imbalance when we are constantly under stress, such as a flood of cortisol and adrenaline in addition to impaired immunity. Learn more about stress management in our Lifestyle module.
Nutrient deficiency and other drivers
A poor diet and poor nutrient absorption from gut permeability can be driving factors in triggering autoimmunity. Some vitamin deficiencies also can increase the likelihood of autoimmune conditions. A lack of sufficient vitamin D, for example, in those who live far from the equator or in cloudier regions, have less robust immune systems that can trigger the onset of autoimmune diseases.
Hormonal imbalances and air pollution are other big triggers that can shift the expression of the genome to create diseased states. (15)
Multi-factorial influence
If it sounds to you like there are number of factors that can affect how our genome expresses, you’d be right. If it sounds like most of these negative epigenetic triggers are a product of our modern world, you’d be right again. While ancient humans still had to deal with stress, smoke from fires, and toxins in their environment and food supply, they had an advantage in that they had far more natural and nutrient dense food choices, a circadian rhythm that led to deep, restful sleep each night, and robust immune systems that were able to fight off infections more easily. So it’s in the very nature of the multiplicity of these factors that our opportunity to reduce and reverse autoimmune disease lies. The closer to nature we can get, the more benefits we can achieve. While we may not be able to control all factors that can be epigenetically triggering, we can learn how to adapt our diet and our lifestyle to reduce negative consequences and continue to live long, healthy lives in spite of autoimmune risk.
Summary
The core takeaway from all of this information is simple: when you know your genome, when you know where your weak points are, you can work on correcting, supporting, and/or supplementing them. You can target your epigenetic switches proactively to help your body to heal, to actually grow healthier over time, and you can do so by modifying the choices you make in your daily life. Using biomarkers to see if damage has already been done and which systems need the most attention is the next big step, since these simple tests can tell you what exactly is going on in your body in real time. When you begin to see how much control you have over your own health, you’ll become more empowered to make the choices that can lead to greater wellness and longevity.
References
- https://ghr.nlm.nih.gov/gene/MTHFR
- https://www.livescience.com/37247-dna.html
- https://www.whatisepigenetics.com/what-is-epigenetics/
- https://snpedia.com/index.php/BRCA1
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2907875/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1566357/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5356847/
- http://onlinelibrary.wiley.com/wol1/doi/10.1111/cdev.12824/abstract
- https://www.hindawi.com/journals/bmri/2015/237183/
- https://www.amymyersmd.com/2017/02/broken-heart-part-1/
- https://www.amymyersmd.com/2017/07/what-is-an-mthfr-mutation-and-what-to-do-about-it/
- https://www.thepaleomom.com/genes-know-mthfr/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078648/pdf/nihms-259545.pdf
- https://healthunlocked.com/nras/posts/134638719/for-all-autoimmuners-free-7-part-documentary-with-tom-obryan
- https://www.thepaleomom.com/what-is-leaky-gut-and-how-can-it-cause/


