What is the microbiome?
The microbiome refers to all the microorganisms that live in a host community. These microscopic organisms are too small to be seen by the human eye and include bacteria, archaea, fungi (yeast), protists, viruses and microscopic animals. (Microscopic animals include mites). These organisms can form a community on or in a larger host organism. The microbiome varies from one animal to another and even within the human population, there can be variability from one human to the next. And even within one human body, there can be variability depending on the part of the body, age, genetics, and environmental influences.
The human microbiome
The human body is full of microorganisms! And most of them are supposed to be there. People are now starting to hear more about the bacteria that live in our gut as being important. Just do a search for microbiome books and you are presented with a wide selection of titles from The Microbiome Solution by gastroenterologist Dr. Robynne Chutkan, MD, to Brain Maker by neurologist Dr. David Perlmutter, MD. But the gut microbiome also includes other organisms besides bacteria, and the human body as a whole has regional microbiomes in other parts of the body, such as the skin microbiome, the mouth microbiome, respiratory microbiome, and the vaginal microbiome. The types of organisms that reside in each of these places differ, serving distinct roles for that region of the body. The Human Microbiome Project is an ongoing research initiative to learn more about the several hundred and even thousands of bacteria and other organisms that populate the human body. The University of Utah has a great interactive website for learning more about the human microbiome.
The gut microbiome
For the purposes of explaining the 5R approach, we will focus mainly on the gut microbiome. Dr. Gerry Mullin, a gastroenterologist from Johns Hopkins Hospital shares some facts about the microbiome. The gut microbiome is a 3-5 pound mass of organisms that reside mostly in the large intestine. Some refer to it as another “organ” as they perform a variety of duties that can benefit our bodies. There are approximately 100 trillion organisms. When compared to the trillion human cells that make up our own body, this means there are more bacteria in the human body than there are human cells! We are outnumbered 10 to 1. Looking at it from this perspective has caused an explosion in the research community to study their function.
The picture below shows the presence of bacteria throughout the gastrointestinal tract with the majority residing in the large intestine (colon).1
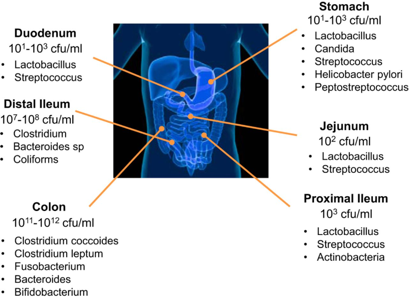
How did all of these bacteria end up in our gut? Before birth, in utero, it is thought that the GI tract is sterile, meaning there are no living organisms. Whether we are born by vaginal birth, or via Caesarian section (C-section), determines our very first exposure to different kinds of bacteria. Breastmilk versus formula feeding further differentiates the types of bacteria we are exposed to. Over the lifespan, the types of bacteria tend to change depending on multiple factors. A baby’s microbiome will look completely different from the microbiome of an elderly person. Everything impacts the organisms that live in our gut. Much of the bacteria we have comes from ingestion. Foods that have been fermented, such as yogurt, kefir, sauerkraut, and others were fermented by bacteria and/or yeast. Bacteria is microscopic — it’s everywhere — and it is no surprise that we are constantly ingesting bacteria. And this is good!
So what do these bacteria do? Why are they there? It turns out that they are vital to our health and well-being. Dr. Mullin explains that the organisms, “break down complex carbohydrates, produce vitamins and nutrients, produce short chain fatty acids (SCFA), protect against pathogens, help train the immune system, support detoxification, and modulate the nervous system.” The nervous system connection is fascinating, and this gut-brain connection is discussed in the “Rebalance” section of the 5R approach. It turns out that the microbiome is a key player, having a “mind of its own”.
What is dysbiosis?
Dysbiosis is an imbalance of the gut microbiome. The imbalance can result from chronic stress, poor dietary intake, antibiotics, infections and toxins such as alcohol. The consequences resulting from an imbalance can involve inflammation, irregular bowel movements, intestinal permeability, nutrient malabsorption and negative food reactions.
Dr. Mullin lists the conditions associated with microbiome imbalance: “Autism spectrum disorders, schizophrenia, depression, anxiety, ADHD, other behavior and mood disorders, migraine headaches, arthritis, cardiovascular disease, cancer, asthma, allergy, diabetes, diseases of the liver, metabolic syndrome, obesity, the 50 million Americans suffering from fibromyalgia, chronic fatigue syndrome, and other autoimmune disorders, and dozens of intestinal disorders that collectively afflict 60-70 million Americans such as irritable bowel syndrome (IBS) have all been associated with an imbalanced microbiome.”
Dysbiosis can lead to leaky gut. The leaky gut connection with autoimmune disease is discussed in the “repair” section of the 5R approach.
SIBO, a form of dysbiosis
Small Intestinal Bacterial Overgrowth (SIBO) occurs when the bacteria of the small intestine overgrow and/or are translocated from another area, such as the large intestine. Dr. Amy Myers describes what causes this condition:
“Damage to the nerves or muscles in the gut can result in leftover bacteria in the small intestine, increasing your risk for SIBO. For example, diabetes mellitus and scleroderma can both affect the muscles in the gut, leaving room for SIBO to develop.
Physical obstructions in the gut, like scarring from surgeries or Crohn’s disease, can also cause an abnormal buildup of bacteria in the small intestine. Diverticuli, which are tiny pouches that can form in the wall of the small intestine, can also collect bacteria instead of passing it on to the colon, where it belongs.
There are also medications that influence or disrupt the normal gut flora, such as antibiotics, acid-blocking drugs, and steroids. And of course, as I mentioned above, the most common cause I see in my functional medicine clinic is from a diet high in sugar, refined carbohydrates and alcohol.”
Dr. Allison Siebecker, ND, a SIBO expert in Portland, OR gives a great overview of the development of SIBO and describes the consequences of having SIBO, including damage to the lining of the small intestine, leaky gut, nutrient deficiencies, and symptoms such as bloating, gas, constipation and diarrhea. In addition to GI-related symptoms, there are also systemic symptoms and associated conditions related to SIBO. Dr. Josh Axe further expands on malnutrition as a complication of SIBO, explaining, “Essential nutrients, protein, carbohydrates and fats aren’t properly absorbed, causing deficiencies, including iron deficiency, vitamin B12 deficiency, calcium deficiency and deficiencies in the fat-soluble vitamins — vitamin A deficiency, vitamin D deficiency, vitamin E deficiency and vitamin K deficiency.”
Many labs offer testing for SIBO, which involves the collection of breath samples in tubes. It is important to keep in mind that treating SIBO is not just about killing off the bacteria. It is also about addressing the factors that set up a person to have SIBO in the first place, so prevention is important. Dr. Siebecker states, “Bacteria can repopulate the SI within 2 weeks of finishing antibiotics, herbal antibiotics or elemental diet, without prevention, though it may take longer.” Treatment options vary depending on the type of bacteria present and Dr. Siebecker does a great job of outlining specific prescription and herbal antimicrobial treatments on her website. Dr. Jill Carnahan also shares her treatment protocol, which involves some different antimicrobial herbal blends. Dr. Axe talks about the importance of probiotics for treating SIBO and that a study “found probiotics have a higher efficacy rate than metronidazole for individuals with SIBO.”
Most functional medicine practitioners recommend a low FODMAPs diet, which is basically a low sugar diet. FODMAPs stands for fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Dr. Axe outlines the low FODMAPs diet and summarizes it into an easy-to-follow chart (you can also see “Eating for Health” for more information).
How do I maintain a healthy microbiome?
Picture your gut microbiome for a moment: trillions of bacteria, along the lining of the mucosal tissue of your large intestine, all responding to the environment around them. How much control do we have over the environment of our intestines? Well, quite a lot, actually. What passes through our gut? Food, medications, beverages, anything else that gets ingested, and whatever gets dumped by the liver. The liver’s role is to detoxify our body. What is surrounding our intestines? Blood vessels carrying neurotransmitters, hormones, nutrients. All of these things affect the environment that house these organisms. We can make the environment a warm and welcome home, rather than a hostile environment. Dr. Gerry Mullin, MD says, “Balance and biodiversity in this ecosystem create health—imbalance and reduced diversity in the ecosystem create illness.”
Multiple studies have shown that diet has the largest influence on what shapes the microbiome. Research points to a Mediterranean Diet as being associated with a healthier microbiome compared to the Western (American) diet.2 Experts like Dr. David Perlmutter, MD and Dr. Alessio Fasano, MD, stress the importance of diet in the health and diversity of the microbiome. Dr. Fasano tells us, “Nutrition, among all the influential elements that can change the microbiota composition, is the key element. True we are using a lot of antibiotics, true that there are a lot of infections, true that C-section delivery is much more popular than in the past, true we travel a lot and therefore we live in a different world, but nutrition, among all the elements, is the most influential to shape our microbiome. After all this is an ecosystem, a parallel civilization that lives in our gut. And they need to eat. And they are fed the leftovers of what we put in our mouths.” The section on pre- and probiotics goes into more detail about types of foods to choose for a healthy microbiome. A brief search online for cookbooks that support a healthy microbiome yields multiple results.
Dr. Perlmutter is often asked how long it takes for the gut microbiome to change with dietary modifications. He quotes a study that showed that just three days after making a dietary change, the microbiome had shifted profoundly.
Pre- and probiotics
Simply put, a probiotic is bacteria that beneficially impacts health, and a prebiotic is the food that feeds these bacteria. Both are necessary to have a robust, diverse microbiome. Pre- and probiotics naturally occur in food sources, but there are also supplements available.
Dr. Gerry Mullin, MD advises to, “Choose nutrient dense prebiotic foods that help the growth of a biodiverse gut microbiome. A few examples are apples, asparagus, artichokes, beans, garlic, leeks, root vegetables, and other foods rich in fiber. Foods that contain live bacteria also bolster the friendly gut flora. Examples of fermented foods that contain healthy friendly bacteria include; kefir, yogurt, miso, sauerkraut, pickled vegetables, kimchi, and more.” Dr. David Perlmutter, MD wrote a great article outlining probiotic and prebiotic containing foods. The process of pasteurization kills bacteria, including the beneficial organisms that helped to culture the product in the first place. Some yogurt companies add beneficial bacteria back in after the pasteurization process. You can also make your own fermented or cultured foods with kits, and that way you don’t have to wonder whether the grocery story item contains viable organisms or not.
Probiotics
For a great overview on prebiotic and probiotic supplements, Dr. Stephen Olmstead, MD, Chief Medical Officer of Klaire Labs, a supplement company that carries probiotics, discusses multiple species in a one-hour webinar. Over a thousand different species of bacteria have been identified that are living within our GI tract, and some have been identified as beneficial, and have been made into supplement form. These bacteria are “friendly”– they encourage the growth of other important bacteria. Probiotics also exert a beneficial effect on the environment they are in, i.e. the gut. They beneficially affect the immune system and there are numerous studies showing the positive impact of probiotics in multiple disease states.3 There are prescription probiotics available through pharmacies, those that can be ordered through a practitioner, and probiotics that can be found over the counter in grocery stores, drug stores, and health food stores.
Probiotic selection. Most probiotics contain a combination of different species of 2 main organisms, Lactobacillus and Bifidobacterium. There are hundreds of species of Lactobacillus alone. These are not the only two beneficial bacteria, they just happen to be the best-studied organisms. There are multiple other probiotic organisms that have a beneficial impact on symptoms. There are probiotics that contain soil-based organisms that do not contain Lactobacillus or Bifidobacterium species. These probiotics are also known as spore-forming bacteria that have similar beneficial effects on the immune system. Dr. David Williams, DC provides a nice table of common probiotics and the health benefits they confer.
Saccharomyces boulardii is a beneficial yeast that is often added to probiotic formulas or given separately. It essentially has similar beneficial impacts on the gut as the probiotic bacteria. Dr. Olmstead tells us it is a transient yeast that antagonizes Candida albicans overgrowth and Clostridium difficile infection, as well as the parasites Entamoeba histolytica, Giardia and Blastocystis hominis.
The United States Food and Drug Administration does not currently allow for probiotics with E. coli nissile, but it is available in most other places in the world.
When asked what probiotic is best during a webinar Q&A, Dr. Robert Rountree, MD of Boulder Wellcare in Boulder, CO says it is not an exact science. He tries different brands with different patients. We are only at the very beginning of understanding the hundreds of different species of bacteria in our gut, and how they interact with each other and with the environment around them. Generally, functional medicine practitioners will choose a multi-strain, multi-species probiotic. Dr. Olmstead may be an expert on probiotic formulations, being that he works for a supplement company that carries multiple probiotics. He says the science points to the benefit of using a multi-species probiotic, and that it is a marketing claim to use single-strain probiotics. Using a multi-species probiotic encourages diversity. Consult with your practitioner for options.
Dosage. Doses of probiotics vary widely. There are products that deliver more of a maintenance dose for regular usage and there are products that deliver therapeutic doses for use while on antibiotics, or for conditions such as Ulcerative Colitis. Doses are reported in CFUs, or colony forming units. A lower dose probiotic may contain a few billion CFUs. A therapeutic high dose can be several hundred billion CFUs. Dr. Amy Myers, MD describes different indications for the different doses of probiotics that she recommends in her practice.
There are differing opinions on multiple aspects of probiotic supplementation:
- Refrigeration or not? The idea behind refrigeration is that these are live organisms and the population is preserved in a cool environment. Whereas heat can kill them off, rendering a supplement that has a bunch of dead bacteria. Some probiotics are shelf-stable and do not need to be refrigerated, while others do require refrigeration. The container will usually say whether it needs to be refrigerated or not.
- Empty stomach or with food? Perlmutter says to take it on an empty stomach because the hydrochloric acid that is released during digestion of food destroys bacteria. Dr. Robert Rountree, MD says he recommends with food. Dr. Stephen Olmstead, MD says with food is best because the pH of the stomach with food is higher and the probiotic will survive it better. Dr. Myers says with or without food is fine.
- Probiotics given with antibiotics or after the course is completed? People wonder if taking a probiotic after having been prescribed antibiotics is even worth it, because isn’t the point of the antibiotic to kill bacteria? Is it just flushing money down the toilet to take a supplement that will be rendered ineffective by the drug? The research actually shows benefit to taking probiotics during a course of antibiotics. Most functional medicine practitioners give high-dose probiotics. Dr. Olmstead says to take probiotics an hour before or 2 hours after the antibiotic. He recommends taking a probiotic for 30 days, meaning the probiotic dosing will continue after the usual 1-2 week course of antibiotics.
Furthermore, as mentioned earlier, Saccharomyces boulardii is a probiotic yeast that will be unaffected by an antibiotic, so a formula containing both bacteria and this yeast would be beneficial. And since yeast balance can be negatively affected by antibiotic use, it’s best to be proactive.
- Probiotics for SIBO or not? Being that SIBO is an overgrowth of bacteria, there is debate as to whether more bacteria should be given. Dr. Myers recommends a soil-based organism type of probiotic for patients with SIBO that does not contain the usual Lactobacillus or Bifidobacterium Dr. Josh Axe, DC quotes a study that showed benefit of giving a mixed probiotic containing various Lactobacillus, Bifidobacterium and Streptococcus faecalis species for patients with SIBO. It seems that taking a probiotic is okay during SIBO treatment, but perhaps a prebiotic may be too irritating or promote symptoms as discussed in the section on prebiotics.
Prebiotics
The bacteria that make up our microbiome require food. Prebiotics are essentially indigestible fibrous carbohydrates that serve as food for the bacteria. Humans cannot digest them, but the bacteria can use them and convert them into byproducts that benefit the human. Some probiotic supplements also have a prebiotic added to the formula. Sometimes prebiotics and prebiotic foods can cause gas and abdominal discomfort, so as with the introduction of any type of fiber, starting slow, and low dosing is recommended. Dr. Doni Wilson, ND says people who should avoid prebiotics include those, “who react negatively to probiotics, fiber and/or FODMAPs. You may need to start by healing your digestion and perhaps taking probiotics prior to adding in prebiotic.”
Examples of prebiotics:
- Acacia gum: David Perlmutter suggests using acacia gum which seems to be better tolerated as compared to inulin or fructose oligosaccharides.
- Arabinogalactan: This is a constituent of the Larch tree, and in addition to being a prebiotic, it also imparts immune system benefits. Dr. Rountree recommends 1 teaspoon to 1 tablespoon of the powder per day.
- Inulin: Inulin is a fibrous substance found in bananas, onions, garlic, chicory, artichokes and asparagus. Dr. Joseph Mercola, DO discusses the health benefits of inulin and says, “If you decide to supplement, begin with no more than 2 to 3 grams a day for at least 1 to 2 weeks. Then, slowly increase your intake by 1 to 2 grams a week until you’re taking 5 to 10 grams a day. Most of the studies used 10 to 30 grams per day, gradually increasing over time.”
- Fructoligosaccharides (FOS): FOS are sugar molecules that the body cannot digest and inulin is a FOS. Often times you will see FOS added to probiotics.
What if probiotics and healthy foods aren’t enough?
Think about a garden. If you have poor quality soil, nothing is going to grow, except maybe weeds. If you enrich the soil, your plant yield increases. This is the same scenario as the microbiome. If you have an unhealthy GI tract, the good guys aren’t going to grow, but the bad guys might. The terrain must be healthy. Why is it that a GI bug goes around and one person becomes ill and another is unaffected? Well, what is the terrain like that is receiving the infectious organism? Is it built with the body’s natural defenses or is it weakened? The 5R approach helps to build a healthy terrain as well as a healthy microbiome.
In some circumstances though, some practitioners are turning to a more involved treatment called FMT as another option to help with reinoculating the microbiota.
Is FMT safe?
Fecal microbiota transplant (FMT) involves inoculating the intestines with someone else’s intestinal bacteria. Stool is transplanted via retention enema, colonoscope or nasogastric/nasoduodenal tube.4 Capsules for ingestion are also now available, according to Open Biome, a stool bank for FMT.
Dr. Gerry Mullin, MD explains, “The Federal Drug Administration (FDA) has approved the use of FMT only for Clostridium difficle infection (CDI) that is refractory to standard medical therapy. This intervention has a cure rate of over 85% as the transplanted microbes outcompete the pathogenic bacterial strains and become members of the recipients gut ecology.” He goes on to say that, “The safety of fecal transplantation has never been formally investigated long term, and clinicians have expressed concerns about FBT “opening up a can of worms” after 4 of 77 patients developed a de novo autoimmune disease after FBT. Furthermore, the FDA limited the practice of FBT to those with CDI-associated diarrhea that failed conventional medical therapy provided donors are properly screened and patients are informed that fecal transplants are still experimental.”
While Dr. Mullin may be conservative with use of FMT for diseases other than CDI, other clinicians like Dr. Josh Axe, DC discuss its use for a wide range of other diseases in a very comprehensive article on FMT. The use of FMT for other conditions is being studied including Inflammatory Bowel Diseases such as Crohn’s Disease or Ulcerative Colitis, although patients are already utilizing this therapy regardless of approval status. Dr. Rountree said that he does recommend fecal transplants for IBD. He said fecal transplant is replacing a whole ecosystem and has a great impact versus giving probiotics.
FMT is safest when the donor stool is screened properly. It is evaluated by a lab and must be tested for pathogenic organisms first in order to qualify as safe. Open Biome is a stool bank that has strict criteria for screening their donors including a 109-point clinical questionnaire, and more than 2 dozen blood and stool screens. In one case of FMT, as explained by Dr. Mullin, a patient gained weight after receiving FMT from her overweight daughter as her donor. This shows the amazing connection between our microbiome and physiological processes that occur outside of the gut, and thus the importance of including a clinical screening as part of the initial process. There is a great interview by Dr. Lawrence Brandt, MD who when asked about contraindications says, “At present, I do not think there are any patients in whom fecal transplantation is contraindicated. I have performed several fecal transplantations in immunocompromised patients without adverse effects. Fecal transplantation therapy is a safe, highly effective, and simple technique that has very few downsides.”5
What about antibiotics?
Antibiotics are frequently prescribed for ear infections, sinus infections, oral infections, urinary tract infections, lung infections, and so on. While they may address the current bacterial infection, antibiotics are not very specific. Meaning, they are also capable of killing much of the bacteria that make up the microbiome, leading to dysbiosis. After a round of antibiotics, the microbiome has to recover and this process can take time. Dr. Aviva Romm tells us, “They are given to about 30% of all women during pregnancy or labor, and these reach the baby through the mom’s blood stream. By 2 years old, 69% of children in the US have received at least one antibiotic course, and the average is 2.3 courses, for ear infections, bronchitis, sore throat, and other common childhood illnesses. By the time our children are 18 years old, they’ll have taken between 10 and 20 courses of antibiotics, and then another 10-13 rounds of antibiotics in their 20s. So by the time we’re 30 years old, on average, we’ve typically had about 30 rounds of antibiotics.”
It’s a wonder whether our microbiome ever truly can bounce back. The Centers for Disease Control report that 1 in 3 antibiotic prescriptions is unnecessary and there is an initiative to stop overprescribing, especially for conditions that probably would have resolved on their own. Furthermore, the problem with antibiotic-resistant bacteria can leave us without options for treatment when an antibiotic really is needed.
Besides taking antibiotics for our own health conditions, Dr. Romm makes us aware of other possible sources: “We’re not just getting antibiotics that are prescribed to us by our doctors. Antibiotics are widespread in our foods, especially animal products. If you drink 2 glasses of milk each day, unless from antibiotic-free animals, you are potentially getting a daily dose of about 50 micrograms of tetracycline. At least 80 million pounds of antibiotics go into our cattle, poultry, and farmed fish each year not just to keep them “healthy” in their over-crowded feedlot conditions, but to promote their growth.”
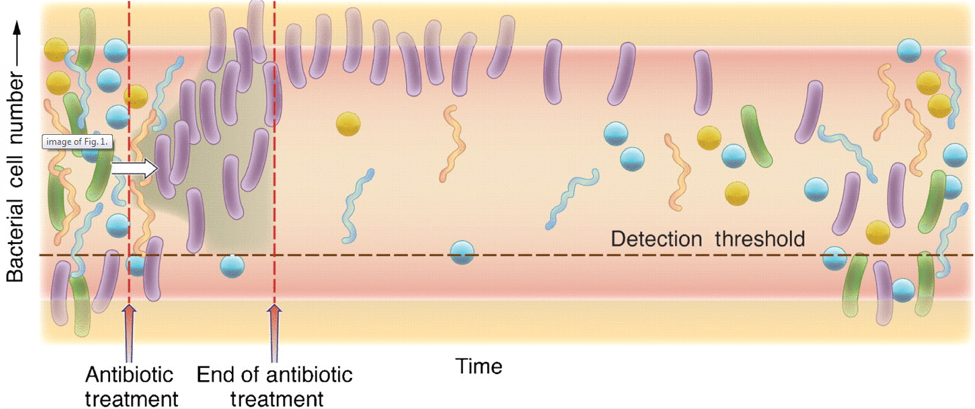
This picture shows a cartoon representation of the microbiome after a course of antibiotics. The left of the diagram shows a diverse, robust healthy population of bacteria represented by the different colors and shapes. As the antibiotic is administered, the presence of the purple rod-shaped bacteria take the opportunity to colonize, crossing the detection threshold, and represents the increase of antibiotic-resistant bacteria. When the antibiotic treatment has ended, there are high levels of antibiotic-resistant bacteria, and low levels of the commensal bacteria that should normally be present in robust amounts, as seen before administration of the antibiotic. As time progresses, moving towards the right of the diagram, you can see that the normal bacterial population begins to increase and diversify again.6
To add insult to injury, while the bacteria of the microbiome are being diminished, there is nothing to keep yeast in check, which can result in yeast overgrowth. Just like in the picture above where the antibiotic resistant bacteria took over, yeast is considered an opportunistic organism that will take its opportunity to move in when there is nothing holding it back. As we have learned throughout this course, yeast can contribute to leaky gut and other symptoms.
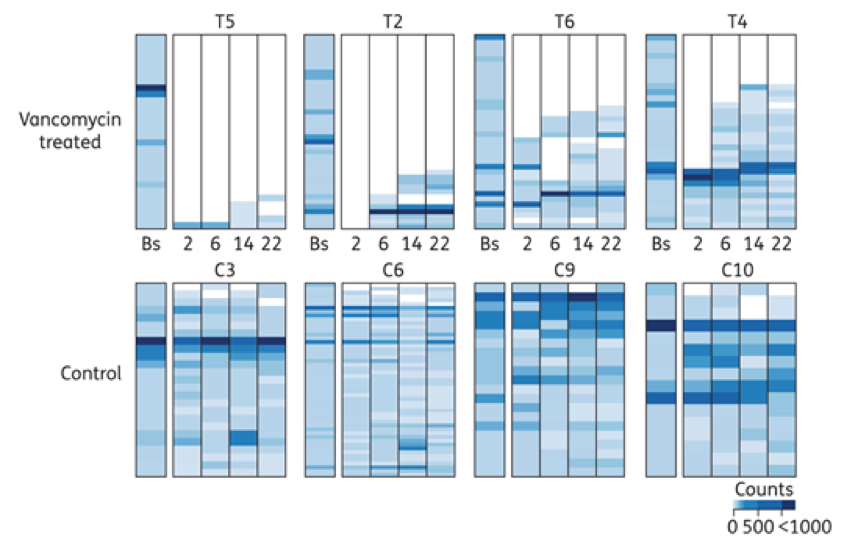
This graph shows 8 people, 4 treated with the antibiotic vancomycin, at the top, and 4 controls that were not treated with an antibiotic, at the bottom. The stack bar grouping for each patient represents the abundance and types of bacteria present at baseline (Bs) and at 2, 6, 14 and 22 weeks. In the 4 vancomycin treated patients, there is a steep decline in both abundance and diversity of the bacteria following treatment as compared to their baseline. This does not appear to recover to the original state, even at 22 weeks. The control groups have a stable abundance throughout the 22 weeks, with minor, normal fluctuations in the diversity, or types of bacteria present.7
Does this mean antibiotics must be avoided at all cost? The options need to be weighed here. This is an important discussion to have with your healthcare provider to learn the consequences of taking versus not taking the antibiotic. If the condition can be addressed via other methods by a skilled practitioner of functional medicine, than this would be a more ideal route. However, not taking an antibiotic for something that could result in negative sequelae or develop into a serious or life-threatening condition is not worth the risk. As the body heals over time and the immune system becomes stronger, the tendency to acquire diseases warranting an antibiotic will decrease. Initially, it may seem like taking the antibiotic is setting you back, but it still may be the best choice considering the circumstances.
Furthermore, many functional medicine practitioners prescribe antibiotics for stealth infections that may be lurking and contributing to chronic illness, such as Lyme Disease. Sometimes antibiotics may be the best treatment, and as long as you are addressing all of the other steps in the 5R approach, you can offset the detrimental effect of the antibiotic. Dr. Amy Myers, MD gives practical tips for when you do take antibiotics, which is basically a 5R approach with liver support. To reiterate from earlier what Dr. Fasano tells us, “Nutrition, among all the influential elements that can change the microbiota composition, is the key element. True we are using a lot of antibiotics, true that there are a lot of infections…” Sometimes treating with antibiotics will get to the underlying root cause of chronic illness. Once that problem is treated, the body can truly heal. In this case, antibiotics may be an ally when you look at the big picture. A temporary disruption in the microbiome with antibiotics, while at the same time having tools to minimize that impact, may be worth it in the long run.
Many functional medicine practitioners utilize antimicrobial herbs instead of antibiotics and there are many studies showing equivalent or better efficacy of many botanical substances for a variety of infections. However, the herbs, like antibiotics, do not discriminate – they also have the potential to kill much of the microbiome. The benefit of herbs over antibiotics is that many herbs have more than one function. The job of an antibiotic is to kill bacteria, although some antibiotics also happen to have an anti-inflammatory effect, which is why they seem to reduce symptoms in viral infections. They are not actually killing the virus, but they are decreasing inflammation, which decreases symptoms, and the person feels better.8 Many herbs are cross-functional and have other beneficial properties in addition to killing microbes.
The best measure is to try to prevent the conditions that would set one up for needing antibiotics in the first place. Are these conditions always avoidable? No, but as we become healthier overall, the body becomes stronger and more nourished and better able to fight infections. Working with a functional medicine practitioner will help to determine the need for an antibiotic or not.
Microbiome Testing
There are a few companies that offer polymerase chain reaction (PCR) testing that measures the DNA of some of the bacteria that make up the commensal bacterial microbiome. There is still much to be learned about the microbiome and these PCR tests are an initial entry into a realm of science that is really only in its infancy. Still, the results may be useful to guide therapeutics. Keep in mind, a unique probiotic formula cannot yet be tailored based on a set of test results. These tests can offer broad generalizations versus specific bacterial recommendations. Some test recommendations do include some specific bacteria, however it is impossible to only alter one part of the microbiome without influencing other parts.
- American Gut is a crowdsourced website that is direct to consumer for research purposes
- Diagnostic Solutions Laboratory offers the GI-MAP
- Genova Diagnostics offers the GI Effects Comprehensive and Microbial Ecology Profile
- UBiome offers the SmartGut test
Conclusion
The microbiome is a hot topic in research and now we can see why. The variety of functions that these microorganisms provide should make us realize that we as humans are truly dependent on these organisms to sustain our lives and our health. In turn, we should work together with them to sustain their health. In the “Rebalance” section of the 5R approach, we will learn more about the microbiome and its influence on our nervous system and psyche; it may have more control over our emotions than one might think. All the more reason to adhere to a diet and lifestyle that creates a happy, healthy microbiome.
References
- Greiner AK, Papineni RVL, Umar S. Chemoprevention in Gastrointestinal Physiology and Disease. Natural products and microbiome.American Journal of Physiology – Gastrointestinal and Liver Physiology. 2014;307(1):G1-G15. doi:10.1152/ajpgi.00044.2014.
- De Filippis F, et al. High-leveladherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016 Nov;65(11):1812-1821. doi: 10.1136/gutjnl-2015-309957. Epub 2015 Sep 28.
- DriskoJA, Giles CK, Bischoff BJ. Probiotics in health maintenance and disease prevention. Altern Med Rev. 2003 May;8(2):143-55.
- Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile Infection with Fecal Microbiota Transplantation.Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(12):1044-1049. doi:10.1016/j.cgh.2011.08.014.
- Brandt LJ. Fecal Transplantation for the Treatment ofClostridium difficile Gastroenterology & Hepatology. 2012;8(3):191-194.
- Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010 Nov;156(Pt 11):3216-23. doi: 10.1099/mic.0.040618-0. Epub 2010 Aug 12.
- Isaac S, Scher JU, Djukovic A, et al. Short- and long-term effects of oral vancomycin on the human intestinal microbiota.Journal of Antimicrobial Chemotherapy. 2017;72(1):128-136. doi:10.1093/jac/dkw383.
- Buret AG. Immuno-modulation and anti-inflammatory benefits of antibiotics: The example of tilmicosin.Canadian Journal of Veterinary Research. 2010;74(1):1-10.





